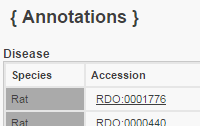
Cellular Component Object Symbol | Species | Term | Qualifier | Evidence | With | Notes | Source | Original Reference(s) | Retsat | Rat | endoplasmic reticulum membrane | located_in | ISS | UniProtKB:Q64FW2 | PMID:15358783 | HGNC-UCL | | Retsat | Rat | nuclear membrane | located_in | ISS | UniProtKB:Q64FW2 | PMID:15358783 | HGNC-UCL | | Retsat | Rat | nuclear outer membrane | located_in | ISS | UniProtKB:Q64FW2 | PMID:15358783 | HGNC-UCL | | | |||||||||||||||||||||||||||||||||||||
|
|
Molecular Function