| ||||||||||||||||||||||||||||
 |
Welcome {{ username}}
Message Center
{{ messageCount }} Messages
Watched Genes
{{$index + 1}}. {{ watchedObject.symbol }} (RGD ID:{{watchedObject.rgdId}})
Watched Ontology Terms
{{gene.symbol}}: {{ gene.description }}
Save List to My RGD
| Create Name: | |
| Description: |
 |
| You must be logged in to use this feature |
| {{ loginError }} |
|
|
{{ watchLinkText }}
Select categories you would like to watch. Updates to this gene will be sent to {{ username }}
| {{geneWatchAttr}} |
Analyze GeneStrainQTL List |
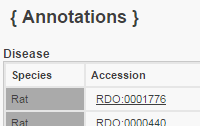 Gene Annotator (Functional Annotation) unavailable |
 Gene Annotator (Annotation Distribution) unavailable |
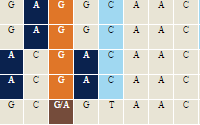 Variant Visualizer (Genomic Variants) unavailble Variant Visualizer (Genomic Variants) unavailable |
Gene Annotator (Functional Annotation) |
Gene Annotator (Annotation Distribution) |
Variant Visualizer (Genomic Variants) |
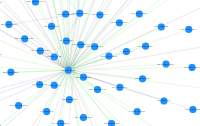 InterViewer (Protein-Protein Interactions) unavailable |
 Gviewer (Genome Viewer) unavailable |
 Variant Visualizer (Damaging Variants) unavailble Variant Visualizer (Damaging Variants) unavailable |
InterViewer (Protein-Protein Interactions) |
GViewer (Genome Viewer) |
Variant Visualizer (Damaging Variants) |
 Gene Annotator (Annotation Comparison) unavailable |
 OLGA (Gene List Generator) unavailable |
 |
Gene Annotator (Annotation Comparison) |
OLGA (Gene List Generator) |
Excel (Download) |
 MOET (Multi-Ontology Enrichement) unavailable |
 GOLF (Gene-Ortholog Location Finder) unavailable |
|
MOET (Multi-Ontology Enrichement) |
GOLF (Gene-Ortholog Location Finder) |







