|
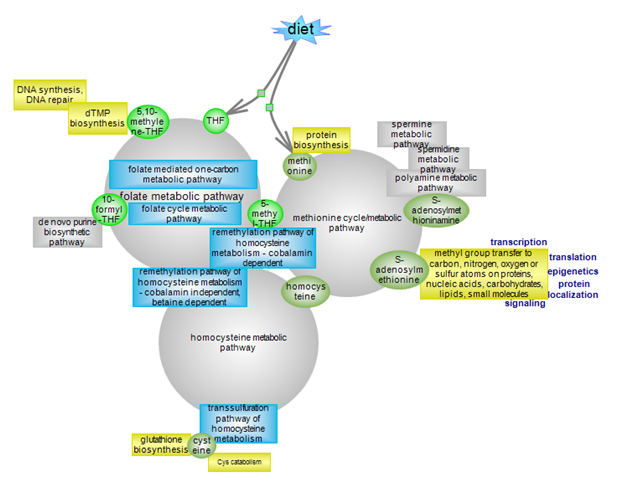
Methionine cycle/metabolic pathway
|
Homocysteine metabolic pathway
|
Folate cycle metabolic pathway
|
Folate mediated one-carbon metabolic pathway |
|||
| The methionine cycle, via the de novo arm, produces the primary methyl donor AdoMet for the transmethylation of proteins, nucleic acids and other molecules, with far-reaching regulatory roles. Along the route it also yields homocysteine whose own metabolism is at the crossroads of several pathways. The salvage arm of the methionine cycle leads to the decarboxylated form of AdoMet, S-adenosylmethioninamine, which is used in the biosynthesis of spermidine and spermine polyamines. Click here to explore the details of the methionine cycle. |
|
Homocysteine metabolism is at the crossroads of three metabolic pathways. Two remethylation pathways regenerate methionine and therefore both its cycle and homocysteine. One route is cobalamin-dependent and requires folate (5-methylTHF) as the one-carbon (1C) donor, the other is independent of cobalamin but depends on betaine as the 1C donor. The third route involves the irreversible degradation of homocysteine to cysteine and further downstream metabolites. Click here to explore the overall aspects of homocysteine metabolism. | The folate cycle and the folate-mediated one-carbon pathways are part of the folate metabolic pathways. The metabolic cycle deals with the various facets involving transport, modifications and interconversions of folates. Click here to explore this critical aspect of folate metabolism. | Tetrahydrofolate (THF) is the co-factor for the generation of 5, 10, or 5 and 10 one-carbon (1C)-substituted biologically active folate derivatives. 5-methylTHF is the 1C donor for the cobalamin-dependent remethylation of homocysteine to methionine. 5,10-methyleneTHF is the 1C donor for the synthesis of thymidylate. 10-formylTHF is the 1C donor for two reactions in the de novo purine biosynthetic pathway, providing the C8 and C2 carbons of the purine ring. Click here to explore this important arm of folate metabolism. |
|
|
||||
|
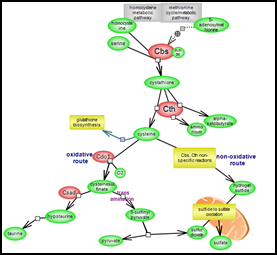
Transsulfuration pathway of homocysteine metabolism
|
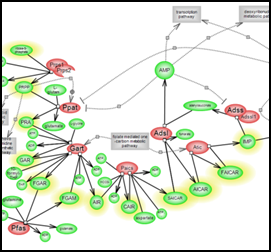
de novo purine biosynthetic pathway
|
|
||
| When the levels of methionine/AdoMet are high, homocysteine metabolism is channeled into the transsulfuration pathway. The cysteine thus derived can be used for the biosynthesis of glutathione, a potent antioxidant. Alternatively, it can be further catabolized into end products that can be fed into other pathways or be excreted. Click here to explore this important aspect of homocysteine metabolism. |
Purines play important roles in nucleic acid biosynthesis, the generation of high energy molecules such as ATP, as constituents of metabolic cofactors like NAD, FAD and coenzyme A and as signaling molecules engaging purinergic receptors. Click here to explore this vital metabolic pathway. |
The polyamine cations interact with nucleic acids, proteins and other molecules and play important roles in cell growth, proliferation and survival. Mammalian polyamine metabolism starts with putrescine, which is derived from L-ornithine, also an important component of the urea cycle. Reactions lead to the formation of higher polyamines, which are converted back to putrescine, easier to excrete from cells. Click here to explore the important reactions of polyamine metabolism. |
|
|
|